Polarity in Solutions
Polar substances can only mix with another polar compound, and non-polar substances can only mix with another non-polar compound. When you have a non polar substance in a polar solvent, the non polar substance will not dissolve in the polar solvent. Think of it like oil and water, they don't mix.
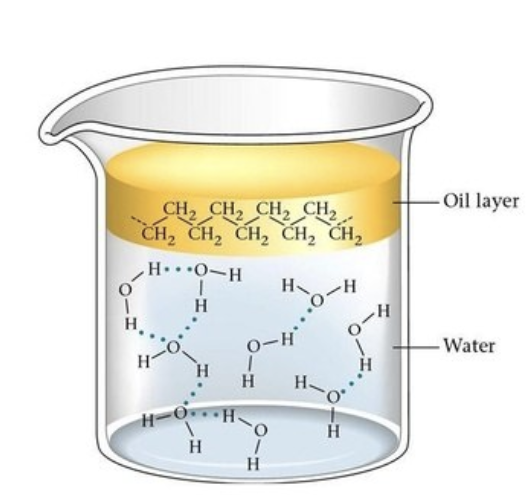
You might of noticed that the example on the right is showing a polar substance refusing to mix with a non polar substance. But the moving compound looks like it should be polar by looking at its size.